participate-birmingham
Participate - University Hospitals Birmingham
Comparing COVID-19 Vaccine Schedule Combinations – Com-COV
THIS SITE IS NOT CURRENTLY RECRUITING
The University Hospitals Birmingham site is recruiting participants from the West Midlands area:
What is the purpose of this research study?
There are now a number of vaccines that have been approved to prevent COVID-19 in the UK. These use two-doses, a ‘prime’ first dose, followed by a ‘boost’ second dose some weeks later. The purpose of this trial is to see how well people’s immune systems respond when they are primed with one type of vaccine, then boosted with another and to see how good the response is when the second dose is separated from the first dose by different periods of time. We will also be looking at how common vaccine reactions, such as fever, are after such ‘mixed’ schedules. This is important, as being able to combine different vaccines in this way, creates a more flexible immunisation programme, potentially allowing more people to be immunised more quickly. It will also give us useful information about extending the gap between prime and boost.
What are the vaccines against?
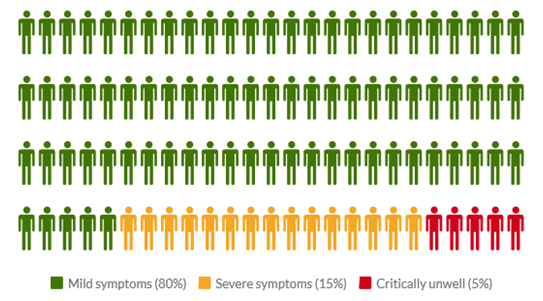
Common symptoms of COVID-19 include fever, tiredness, dry cough, and changes to taste and smell. Whilst about 80% of infected people have no or mild symptoms and will recover from the infection without needing special treatment, approximately 10-15% of cases (2-3 in 20) progress to develop severe symptoms, and about 5% (1 in 20) become critically ill.
There are some treatments that have been shown to be effective in reducing the severity of disease and the risk of death; but at present there is no cure. Older people and those with underlying medical conditions are more likely to develop serious illness. It has also been seen that people of some ethnic groups (Black and Asian) might be at a greater risk of severe illness. More than 1.4 million people globally have died from COVID-19 so far. Some people also have symptoms that last a long time after they have recovered (commonly referred to as “long-COVID”). This is why effective vaccines are so important.
Am I suitable to take part?
Adults that are aged 50 and over are able to take part. In order to be enrolled in the study:
- You must be willing to tell the trial staff about your medical history, and you may be asked to allow the trial staff to check this with your General Practitioner (GP)
- If you are able to become pregnant you must be willing to practice continuous effective contraception during the study and have negative pregnancy tests on the days of vaccination
- You must agree not to donate blood during the study
For further details on why you could not take part in this study please see the Participant Information Sheet.
Summary of the study
We are studying combinations of two different vaccines, and there will be 820 participants. As more new SARS-CoV-2 vaccines become available, more vaccines may be included in the trial and so the total number of participants may increase.
- Participants will be allocated, at random, (rather like a flip of a coin) to receive one dose of one approved vaccine and a second dose of either the same approved vaccine, or a dose of a different approved vaccine. Participants will also be allocated at random to the timing of receiving these doses – some will get a boost dose four weeks after the first dose (prime) and some will get a boost at twelve weeks.
- Between 5 and 9 routine blood tests will be taken over the course of a year to look at the immune responses to the vaccine depending on the group you are in. You may also be asked for a nasal fluid sample at each visit. If you were to test positive for the virus causing COVID-19 we may ask you to attend for an extra visit.
- Participants will need to complete an online diary for up to 28 days following each of the two vaccinations
- The trial will take one year to complete per participant (from the time the first dose of vaccine is given)
- We would not be offering diagnostic COVID-19 testing as part of this trial, but it is important that participants in this trial access COVID-19 testing outside of the trial following normal government guidance.
- If you receive the two doses of COVID-19 vaccines in this trial, you would not know which two vaccines you had received until the end of the trial. Unless specifically advised by us, you would not be eligible to receive any further vaccine doses via the government vaccination scheme.
What vaccines are being used in this study?
At the moment, the two vaccines in this study are AstraZeneca ChadOx1 nCoV-19) and Pfizer BioNTech BNT162b2. If more vaccines are approved for use in the UK after this study starts, they may be added to the trial. You will only ever receive two doses of any vaccine as part of the trial; if new vaccines are added, they would be given to new participants.
Vaccine A - AstraZeneca ChadOx1 nCoV-19
ChAdOx1 nCoV-19 is made from a virus (ChAdOx1), which is a weakened version of a common cold virus (adenovirus) from chimpanzees that has been genetically changed so that it is impossible for it to grow in humans. Added to this virus are genes that make proteins from the COVID-19 virus (SARS-CoV-2) called Spike glycoprotein (S), which play an essential role in infection SARS-CoV-2 infection. By vaccinating with ChAdOx1 nCoV-19, the body recognises and develops an immune response to the Spike protein that will help stop the SARS-CoV-2 virus from entering human cells and therefore prevent infection.
This is the vaccine that is commonly known as the “Oxford vaccine”. It has been tested in more than 20,000 people worldwide as part of the COVID-19 vaccine trials. It has been found to be both safe, and efficacious in preventing COVID-19.
Vaccine B - Pfizer BioNTech BNT162b2
This is a messenger RNA (mRNA) vaccine, a new kind of vaccine. This vaccine uses a small amount of the genetic coding material (mRNA) of the SARS-CoV-2 spike (S) protein packaged inside very small fatty particles (lipid nanoparticles). When these are injected into your body, your cells take up these fatty particles, and then start producing the SARS-CoV-2 spike protein. Your immune system then “sees” these spike proteins, and makes a protective immune reaction against them. The original mRNA that has been taken into your cells is broken down within a few days, and cannot be incorporated into your own genetic code.
This vaccine has been tested in more than 40,000 people worldwide and has been shown to be both safe, and efficacious.
Neither vaccine contains the SARS-CoV-2 coronavirus and therefore cannot give you COVID-19
What are the side effects of these vaccines?
Common side effects
People very often have tenderness, pain, warmth, redness, itching, swelling or bruising or less commonly have a small lump in their arm where they have been vaccinated.
Other common systemic side effects
Some people can develop these symptoms after vaccination. They usually last for less than a week after you are vaccinated (more commonly 24-48hours afterwards).
- Fatigue
- Headaches
- Flu-like symptoms, such as high temperature, sore throat, runny nose, cough and chills
- Muscle aches
- Joint aches
- Feeling unwell (malaise)
- Feeling sick or nauseated or vomiting
Other less common side effects:
- Abdominal pain
- Decreased appetite
- Feeling dizzy
- Swollen lymph nodes (glands)
- Excessive sweating, itching skin or rash
These symptoms can be reduced by use of paracetamol around the time of immunisation and over the next 24 hours.
After immunisation with the BNT162b2 (Pfizer/BioNTech) vaccine, difficulty sleeping has been observed in fewer than 1 in 100 people, and weakness of the muscles on one side of the face has been observed in fewer than 1 in 1000 people.
What are the advantages of taking part?
We anticipate that participating in the trial will mean that you gain some protection against the coronavirus (but cannot guarantee this). It is possible that you could gain this protection sooner than you otherwise would have by waiting for your place in the vaccine roll out. Most importantly, the information gained from the trial will make a valuable contribution to the pandemic response.
Are there any risks from taking part in the study?
In addition to the potential side effects of the vaccines outlined above, the blood samples taken in the study may cause slight pain and occasionally bruising. Please refer to the participant information sheet for full details of procedures and potential risks.
What will happen if I don’t want to carry on with the trial?
If, at any time after agreeing to participate, you change your mind about being involved with this study you are free to withdraw without giving a reason. If you withdraw we would not usually perform any more research procedures, although occasionally we might need to offer you a follow up visit for safety purposes, for example to check the injection site or a blood result. Your decision will not result in any penalty. Unless you state otherwise, any samples taken whilst you have been in the study will continue to be stored and used for research as detailed above. You are free to request that your samples are destroyed at any time during or after the study. If you choose to withdraw from the trial, your standard medical care will not be affected.
What’s next?
If you would like to find out more, please read the Participant Information Sheet and if you are interested in taking part, please complete our pre-screening questionnaire.
Download the Participant Information Sheet (PDF)
THIS SITE IS NOT CURRENTLY RECRUITING
Complete the Pre-screening Questionnaire
For further details contact us on :
This site will open for recruitment soon and contact information will appear here when it opens.




